QUALITY ASSURANCE
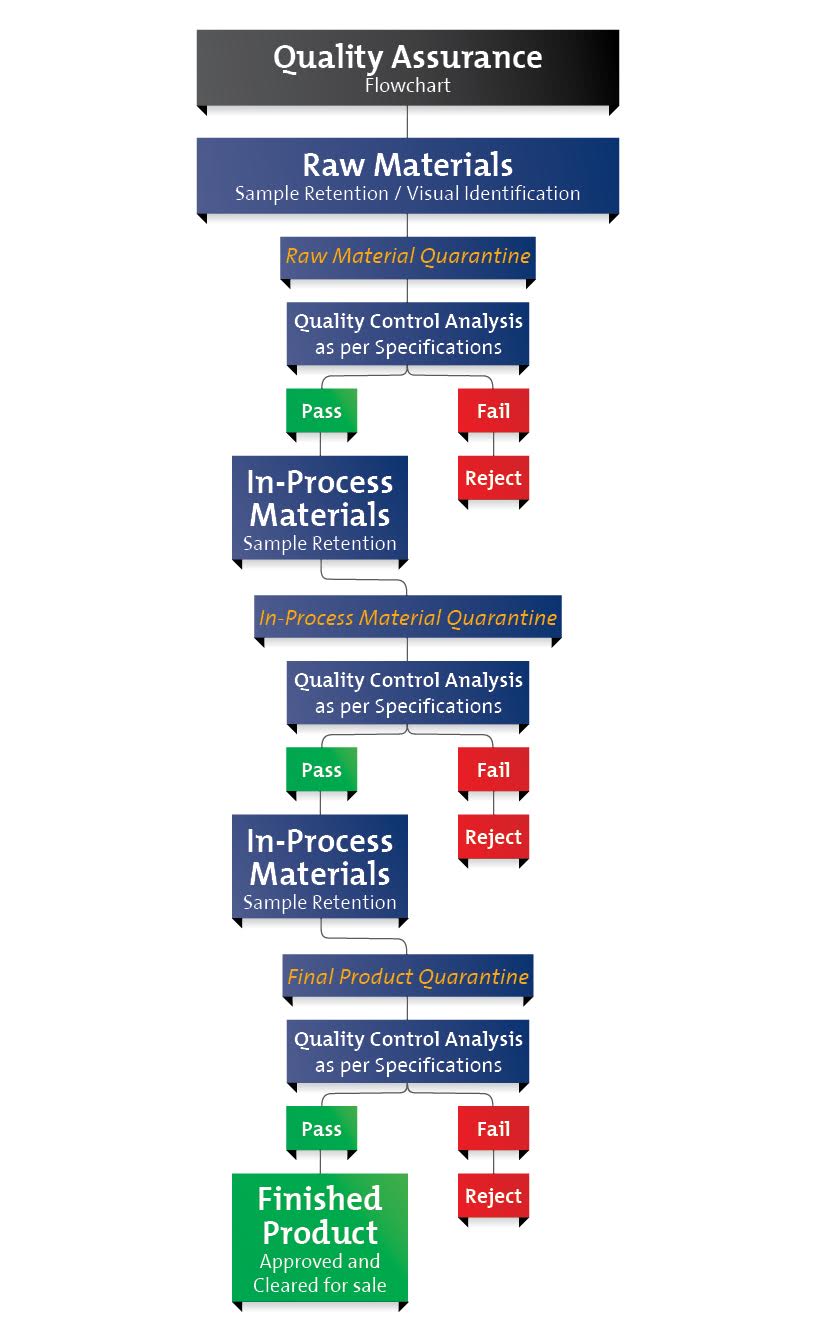
At NFH, we strive to maximize clinical benefit with our evidence-based products and setting the standard in raw material and finished product quality excellence ensures this. All testing is conducted in a third-party ISO 17025 certified laboratory – one of the most advanced laboratories in Canada. All raw materials that enter the facility are tested three times: Upon entering the facility, during processing, and in the finished product stage.
Raw Materials Testing
We surpass the regulations of Health Canada and the FDA by the following:
- We validate every single raw material lot that enters our facility prior to manufacturing – we do not skip lot test.
- All dietary ingredient raw materials that enter our manufacturing facility, regardless of country origin (including the USA and Canada), are quarantined and then subject to the same rigorous testing.
- We do not rely on suppliers certificates of analysis for identity, potency or purity; rather, all raw materials are tested by a third-party laboratory.
- No raw material is released from quarantine and accepted for manufacturing unless it passes all rigorous tests and meets our specifications.
- Certificates of analysis from a third-party laboratory are available for every raw material and finished product lot.
| Test | Method |
|---|---|
| Identity, authenticity, and potency – Utilizing established scientific testing methods and reference standards | Specific to ingredient tested i.e. Tandem Quadrupole UPLC/MS/MS, HPLC, UPLC, GC, GC-FID, GC/MS, GC/MS/MS, FT-NIR, ICP-OES, and UV |
| Microbiology – Total bacterial count, total mold & yeast count, Salmonella species, E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa | USP 61 & USP 62 |
| Heavy metals – Arsenic, cadmium, mercury, methyl mercury, and lead | EPA 6010C using ICP/OES |
| Pesticides – >80 tests for pesticides, herbicides, fungicide, and Polychlorinated Biphenyls (PCB) | EPA 8141A/B, 8080A & 8081A |
| Aflatoxins & mycotoxins | AOAC 993.17 & USP-NF 561 |
| Chemical solvent residues | USP 467 |
| Dioxins and oxidation bi-products (peroxide and anisidines) | Peroxide value: AOAC 965.33 Anisidine value: USP 31<391> Dioxins: EU 252/2012 or USEPA 1613B |
| Disintegration | USP 2040 |

Finished Product Testing
Similar to raw material testing, we validate the identity, potency, and purity of every single finished product lot number prior to leaving our manufacturing facility – we do not skip lot test for finished products. These rigorous standards of quality assurance ensure authenticated content and potency to meet label claims through to expiration and freedom from contaminants with every finished product lot number. From our long history of analytical experience and testing, we have developed programs to validate the stability and expiration date of finished products.
In addition, NFH products have acquired Natural Product Numbers (NPN) from Health Canada. The eight-digit NPN substantiates the product has been assessed and licensed by Health Canada and found to be safe, effective and of high quality under their recommended conditions of use.
Laboratory Equipement
At NFH, we are committed to researching and employing the most scientifically valid and reliable laboratory test methods and reference standards. The most important feature of ISO 17025 is the development of testing methods that are of the highest global standards and latest scientific technology. Our industry-leading laboratory testing utilizes advanced analytical equipments to examine the fingerprint of all plant material constituents versus only a specific marker. This level of sophistication allows us to authenticate the specific genus and species of every raw material, therefore confirming identity as wells as potency and purity for each specific therapeutic compound sourced for our product formulations. With some of the most advanced equipment in the world, we are able to validate over 800 raw materials using the latest analytical methods. Additionally, our rigorous analytical labs recognize seasonal and geographical variability as well as adulterated or spiked raw materials. Ultimately, our sophisticated instruments have greater sensitivity to achieve the most precise test results possible.
Our validated laboratory methods have been adopted from the USP, AOAC, ISO, Health Canada, Ph. Eur, BP, EPA, ASTM, and others as needed.

HPLC’s
High-Performance Liquid Chromatography
The HPLC is a master specialist in separating components in a sample and determining the identity and quantity of elements and molecules based on their physical and chemical properties. HPLC can quantify the activity in an herb, product of an enzymatic reaction, or any molecule able to absorbs or transmit light. Therefore, the HPLC is very specific in determining what a substance is and its exact quantity.
The HPLC is used routinely to analyse the composition of compounds present in complex mixtures, such as water- and fat-soluble vitamins, as well as in the analyses of standardized plant extracts and amino acids.

UPLC/MS/MS (Xevo Tandem Quadrupole)
Ultra Pressure Liquid Chromatography Mass Spectrometer – Dual detector
The UPLC/MS/MS is highly sophisticated and able to do everything the HPLC can do, however, with more accuracy and precision. It does this by using a very high-pressure micro-pump (15,000 psi) combined with dual detector – Photodiode Array (PDA) and a more powerful mass spectrometer detector. The LC/MS combines the advanced separation capabilities of an HPLC with the powerful analytical abilities of a mass spectrometer.
Currently, this is the most advanced and widest application tool for analysis. It allows for the most precise measurements in parts per trillion rather than parts per billion or million. The UPLC/MS/MS is able to effectively analyse herbs and medicinal components. It is used in cases where extreme sensitivity is needed and can also identify numerous actives in a finished product.
The main advantage of this system is that it generates fast, accurate and extremely precise measurements by creating an electronic signature of a compound. We test many nutraceuticals with this instrument such as glycosides in black cohosh, amino-acids, and Ginkgo biloba.

GC-FID/MS
Gas Chromatography-Flame Ionization Detector/Mass Spectrometry
The GC is used to analyse volatile molecules with a high melting point such as all oil-based products, including fatty acids in fish oil. In addition, samples submitted to the GC do not need solvents or a liquid mobile phase. Instead samples are carried by an inert gas through the system. Thus, if we are testing for solvents the instrument of choice is the GC-FID/MS.
GC-MS is precisely able to both identify and quantify the molecules of interest, whereas, GC-FID is only used to quantify molecules, such as common fatty acids and essential oils. It looks at fatty acid profiles to validate the purity of an oil and quantifies its claim (i.e. Evening Primrose Oil, 10% GLA). Like LC-MS/MS, GC-MS is also able to create an electronic signature of a molecule. The complexity of running the test will dictate which instrument will be used.
The GC-MS is also used to test for PCBs, herbicides, and pesticides. Conversely, the GC-FID is used to detect solvents and their residues, such as 1, 2-dichloroethane and 1,1,1-trichloroethane, which are known human carcinogens. These contaminants can be present in low quality herbal extracts and we have zero tolerance for them.

ICP-OES
Inductively Coupled Plasma – Optical Emission Spectroscopy
ICP specializes in analysing metals and minerals. With this device we can effectively and precisely determine the identity and quantity of any metal present in a sample, including iron, magnesium, lead, mercury or boron. The process to test for these metals is much more straightforward than it would be on the HPLC or LC/MS.
We use the ICP-OES to detect contamination by low-level trace metals including mercury, arsenic, lead and cadmium. These contaminants permeate the earth’s crust and can be especially present in foodstuff grown in the ground or any items originating from the earth. Therefore, this instrument validates that raw materials are free from any heavy metals as it has the capacity to detect any element on the periodic table.

Spectrophotometer
The spectrophotometer is a cost effective tool that can be used to determine the quantity of samples that absorb or transmit light. This instrument is used to identify the species of raw herbs. It also detects flavonoids in plant extracts, such as grape seed extract where the flavonoids are colour-based. In addition, it can validate enzymes, such as plant digestive enzymes and animal-based enzymes. However, the identity of the sample will be determined through other instruments.
Our spectrophotometer is used to determine some enzymatic activities, such as papain and bromelain or anthocyanidin content in Bilberry.

HPTLC
High Precision Thin Layer Chromatography
The HPTLC is an effective tool to verify the fingerprint identity of plant material against a reference plate. We are able to confirm the profile of a plant with this tool and ensure the correct raw material is being used.
We use the HPTLC to test for aflatoxins, a group of chemically similar toxic fungal metabolites (mycotoxins) produced by certain molds of the genus Aspergillus that can grow on plant matter in humid conditions, namely raw herbs. Testing for aflatoxins and mycotoxins is critical as they are highly toxic compounds and can lead to both acute and chronic toxicity in humans.

NIR
Near Infrared Spectroscopy
NIR can be used to test a wide variety of substances from raw herbs to isolates, such as amino acids. With this device we can guarantee the freshness of the plants used in our products.
The NIR gives a reliable identification of a sample by comparing its spectra to the spectra of a sample of known characteristics. NIR analyses the transmissive properties of specific wavelengths of light in the sample being measured.
NIR allows us to identify the total quality of herbal products. We ensure that only those samples that meet our strict criteria for freshness and quality are able to pass. We only utilize the highest quality herbs in the reference models we create for the NIR.